Let’s delve into the intricacies of calculating the weight of a water molecule. As a chemistry enthusiast, I find the molecular weight of water fascinating. Understanding the weight of a water molecule can shed light on a variety of chemical and physical properties that are vital to many scientific disciplines. In this blog post, I’ll break down the process of calculating the weight of a water molecule, and explain the significance of this calculation in various fields of study. Understanding the weight of a water molecule is crucial for comprehending its role in chemical reactions and physical processes, making it a fundamental concept in the world of science. Whether you’re a chemistry student or simply intrigued by the mysteries of the molecular world, this post will provide a comprehensive overview of this important calculation.
Key Takeaways:
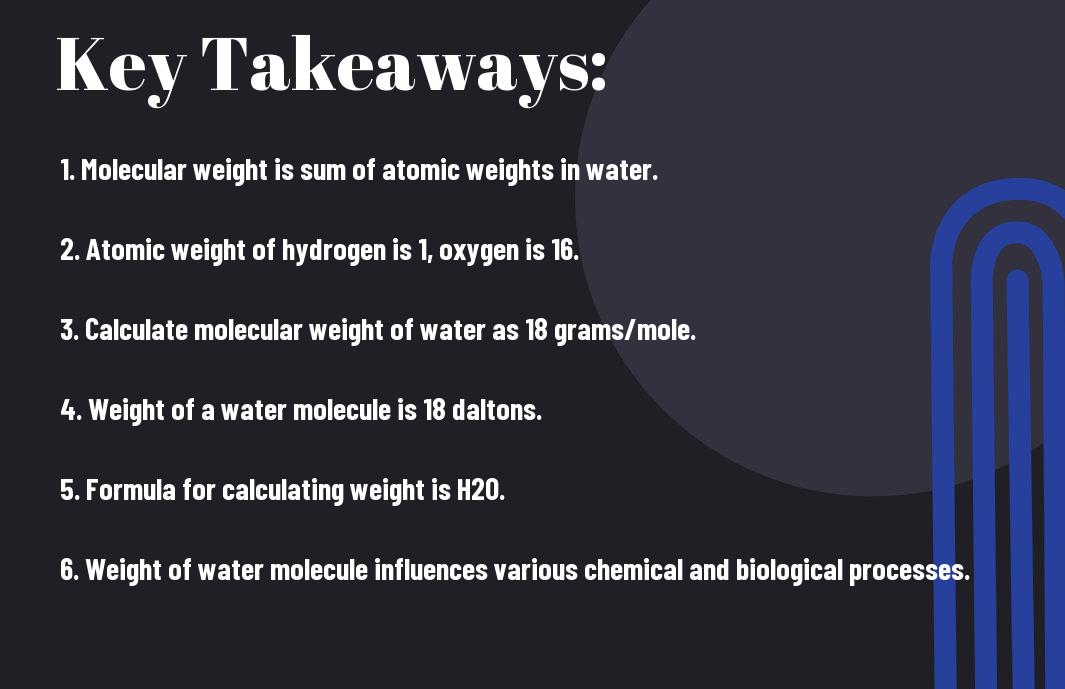
- Water molecule weight: The weight of a water molecule is calculated by adding the atomic masses of its constituent atoms, two hydrogen atoms and one oxygen atom.
- Atomic masses: The atomic mass of an element is the weighted average of the masses of the isotopes of that element, with hydrogen having an atomic mass of approximately 1 and oxygen having an atomic mass of approximately 16.
- Molecular weight: The molecular weight of a water molecule (H2O) is calculated as the sum of the atomic masses of its constituent atoms, which is approximately 18 grams per mole (g/mol).
- Formula weight: The formula weight of a substance is the sum of the atomic masses of the atoms in its chemical formula, which in the case of water is 18 g/mol.
- Importance of molecular weight: Understanding the molecular weight of water is important in various scientific and industrial applications, including chemistry, biology, and environmental science.
The Molecular Weight of Water
The molecular weight of water, also known as the molar mass of water, is the total weight of a water molecule. It is computed based on the atomic weights of hydrogen and oxygen, the two elements that form water.
Calculating the Molecular Weight
The molecular weight of a water molecule is calculated by adding the atomic weights of its constituent elements. In the case of water, the molecular weight is the sum of the atomic weight of two hydrogen atoms and one oxygen atom, giving a total of 18.01528 g/mol.
Factors Affecting the Molecular Weight
Several factors can affect the molecular weight of water. These include the isotopic composition of hydrogen and oxygen, as well as any impurities present in the water sample. The most common form of water, H2O, has a molecular weight of 18.01528 g/mol. However, heavy water, which contains the isotope deuterium, has a molecular weight of 20.0276 g/mol. Impurities such as dissolved minerals can also slightly alter the molecular weight of water. It’s important to consider these factors when conducting precise measurements in laboratory settings.
- Isotopic composition: The presence of isotopes such as deuterium can increase the molecular weight of water.
- Impurities: Dissolved minerals and other substances can alter the molecular weight of water.
After accounting for these factors, the molecular weight of water can be more accurately determined.
Importance of Understanding the Weight of a Water Molecule
Now that we’ve delved into the calculation of the weight of a water molecule, you might be wondering why this information is important. Understanding the weight of a water molecule is crucial for various scientific and practical reasons. Knowledge of the weight of a single water molecule can provide valuable insights into the properties and behavior of water at the molecular level. If you are interested in calculating the mass of a single water molecule in grams, you can find a detailed guide here.
Environmental Impact
Understanding the weight of a water molecule is vital in assessing the environmental impact of water pollution. The weight of water molecules affects the density and behavior of water, which in turn influences its ability to sustain aquatic life and contribute to ecological balance. Additionally, the weight of water molecules plays a critical role in the water cycle and global climate patterns.
Industrial Applications
The weight of a water molecule is essential in various industrial processes, such as chemical reactions, manufacturing, and materials science. Knowing the weight of water molecules is crucial for controlling and optimizing chemical reactions, designing industrial processes, and developing new materials. Furthermore, in fields such as pharmaceuticals and biotechnology, understanding the weight of water molecules is fundamental for research and development.
I hope you find the details of this section useful. If you want me to widen more on any subject, please do not hesitate to contact me.
Methods and Tools for Calculating the Weight of a Water Molecule
Despite the small size of a water molecule, calculating its weight involves using specific methods and tools. One important tool for this is the concept of Molecular Weight, which allows scientists to determine the weight of a water molecule based on the weights of its individual atoms. In addition to this, there are several methods and techniques that can be used to calculate the weight of a water molecule.
Chemical Analysis
Chemical analysis is a crucial method for determining the weight of a water molecule. By breaking down the molecule into its constituent elements (two hydrogen atoms and one oxygen atom), scientists can calculate the combined weight to determine the molecular weight of water. This process is essential for understanding the composition of water and its weight in various chemical reactions or natural processes.
Spectroscopy
Another important method for calculating the weight of a water molecule is spectroscopy. This technique involves the interaction of matter with electromagnetic radiation, allowing scientists to study the structure and composition of molecules. Spectroscopy can provide valuable data on the weight and behavior of water molecules, contributing to our understanding of their physical and chemical properties.
Conclusion
Upon reflecting on the process of calculating the weight of a water molecule, it becomes clear that the formula H2O can be used to determine the molecular weight. The atomic weight of hydrogen is 1, and the atomic weight of oxygen is 16, so by adding these together we get the molecular weight of water, which is 18. This simple calculation helps us to understand the composition and properties of water, and its importance in various chemical and biological processes. Understanding the weight of a water molecule is fundamental to grasping the behavior of water in various environments and its significance in sustaining life on Earth. It is a basic concept in chemistry, but one that is crucial for understanding the world around us.
FAQ
Q: What is the weight of a water molecule?
A: The weight of a water molecule is approximately 18 atomic mass units (amu).
Q: How is the weight of a water molecule calculated?
A: The weight of a water molecule is calculated by adding the atomic weight of two hydrogen atoms (each with an atomic weight of approximately 1 amu) to one oxygen atom (with an atomic weight of approximately 16 amu). This results in a total weight of approximately 18 amu.
Q: What is the significance of knowing the weight of a water molecule?
A: Understanding the weight of a water molecule is essential in various scientific and industrial applications, including chemical reactions, environmental studies, and pharmaceutical research. It also allows for accurate measurements in fields such as chemistry and physics.
Q: How does the weight of a water molecule impact its physical properties?
A: The weight of a water molecule influences its physical properties, such as boiling point, freezing point, and density. For example, the relatively low weight of a water molecule contributes to its ability to exist in all three states of matter at normal temperatures and pressures.
Q: Can the weight of a water molecule vary under different conditions?
A: The weight of a water molecule remains constant under standard conditions. However, changes in temperature, pressure, or isotopic composition can slightly alter the weight of a water molecule. For practical purposes, these variations are generally negligible in most applications.








Leave a comment